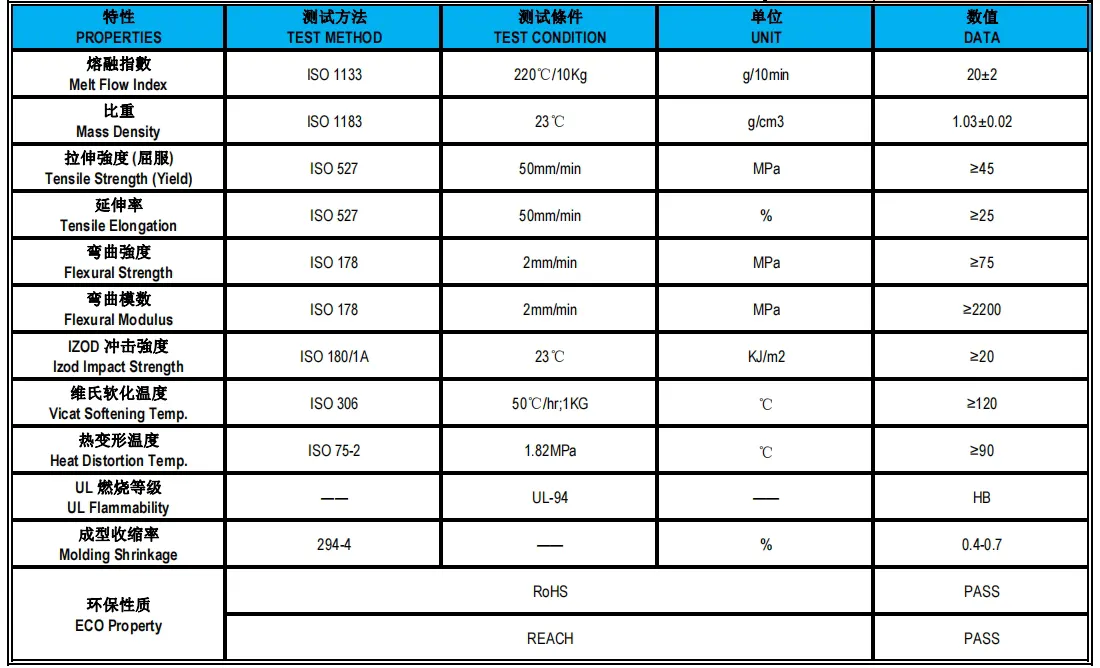
Plastic raw materials glossary - no longer afraid not to understand the physical property table
1. Density and relative density
Density and relative density - Density refers to the mass contained in the unit volume of a substance, in short, the ratio of mass to volume, which is measured in millions of grams per meter 3(Mg/m3) or kilograms per meter 3(kg/m3) or grams per centimeter 3(g/cm3).
Relative density, also known as the ratio of density, refers to the ratio of the density of a substance to the density of a reference substance under their respective specified conditions, or the mass of a certain volume of a substance at t1 temperature and the equivalent volume of a reference substance at t2. The ratio of mass at temperature. A common reference substance is distilled water, expressed as Dt1/t2 or t1/t2, which is a dimensionless quantity.
2. Melting point and freezing point
Melting point and Freezing point - The temperature at which a substance's liquid-solid state reaches equilibrium under its vapor pressure is called the melting point or freezing point.
This is due to the regular arrangement of atoms or ions in the solid due to the rise in temperature, the thermal movement becomes chaotic and activated, forming a phenomenon of irregular arrangement of liquid, the opposite process is solidification. The temperature at which a liquid changes to a solid is often called the freezing point or freezing point, and differs from the melting point in that heat is emitted rather than absorbed. In fact, the melting point and freezing point of matter are the same.
3. Melting range
Refers to the temperature range measured by the capillary method from the beginning of the melting of the substance to the complete melting.
4. Crystal point
Refers to the liquid in the cooling process, from liquid to solid phase change temperature.
5. Pourpoint
An indicator of the properties of liquid petroleum products. Refers to the temperature at which the sample is cooled to begin to stop flowing under standard conditions, that is, the lowest temperature at which the sample can still be poured when it is cooled.
6. Boiling point
The temperature at which a liquid boils when heated and turns into a gas. Or the temperature at which the liquid and its vapor are in equilibrium. In general, the lower the boiling point, the greater the volatility.
7. Boiling range
In the standard state (1013.25hPa, 0℃), the distillation volume within the temperature range specified in the product standard.
8. Sublimation
The transformation of a solid (crystalline) substance into a gaseous state without passing through the liquid state. Such as ice, iodine, sulfur, naphthalene, camphor, mercury chloride, etc., can be sublimed at different temperatures.
9. Vaporizing velocity
Evaporation refers to the gasification of the surface of a liquid. Evaporation rate, also known as volatilization rate, is generally judged by the boiling point of the solvent, and the fundamental factor determining the evaporation rate is the vapor pressure of the solvent at this temperature, followed by the molecular weight of the solvent.
10. Vapor pressure
Vapor pressure is short for saturated vapor pressure. At a certain temperature, the liquid reaches equilibrium with its vapor, and the equilibrium pressure at this time changes only because of the nature and temperature of the liquid, which is called the saturated vapor pressure of the liquid at this temperature.
11. Azeotrope
The constant boiling point mixture formed by two (or several) liquids is called the azeotrope, which refers to a mixed solution in equilibrium, where the gas phase and liquid phase are completely the same. The corresponding temperature is called the azeotropic temperature or azeotropic point.
12. Refractive index (Refractive index)
Refractive index is a physical quantity that expresses the ratio of the speed of light in two different (isotropic) media. The speed of light varies with the medium, when the light from a transparent medium into another transparent medium with different density, due to the speed change, the direction of its change, it is called refraction.
The ratio of the sine of the Angle of incidence of light to the sine of the Angle of refraction, or the ratio of the speed of light passing through a vacuum to that of a medium, is the refractive index. The generally expressed refractive index n refers to the value of light entering any medium by air. The refractive index usually referred to is measured by sodium yellow light (D-line) at tC, so it is expressed by ntD, such as measured at 20 ° C, it is n20D.
13. Flashing point
Flash point, also known as burning flash point, indicates one of the indicators of the nature of flammable liquid. It is the lowest temperature at which the mixture of vapor pressure and air on the surface of the flammable liquid is heated to flash when it comes into contact with the flame. Flash is usually a light blue spark, a flash is extinguished, can not continue to burn.
Flashover is often a harbinger of fire. There are open-mouth cup method and closed-mouth cup method to determine the flash point, the former is generally used to determine the high flash point liquid, the latter is used to determine the low flash point liquid.
14. Ignition point
Ignition point, also known as ignition point, is one of the indicators of the properties of flammable liquids. It refers to the minimum temperature at which the vapor and air mixture heated to the surface of the flammable liquid can continue to burn immediately after contact with the flame. The ignition point of flammable liquid is 1 ~ 5℃ higher than the flash point. The lower the flash point, the smaller the difference between the flash point and the flash point.
15. Spontaneous ignition point
The lowest temperature at which combustible substances can ignite without contact with an open flame is called the spontaneous ignition point. The lower the spontaneous ignition point, the greater the risk of ignition. The spontaneous ignition point of the same substance varies with different conditions such as pressure, concentration, heat dissipation and test methods.
16. Explosive limits
Combustible gas, flammable liquid vapor or combustible solid dust at a certain temperature, pressure and air or oxygen mixed to reach a certain concentration range, encounter the fire source will explode. This concentration range is called the explosion limit or the combustion limit. If the composition of the mixture is not within this certain range, no matter how large the supply of energy, it will not catch fire.
Vapor or dust mixed with air and reach a certain concentration range, encounter the fire source will burn or explode the lowest concentration is called the lower explosive limit; The maximum concentration is called the upper limit of explosion. The explosion limit is usually expressed as a percentage of the volume of the vapor in the mixture, i.e. %(vol); Dust is expressed in mg/m3 concentration.
If the concentration is lower than the lower explosive limit, although the open flame will not explode or burn, because the proportion of air is large at this time, and the concentration of combustible vapor and dust is not high; If the concentration is higher than the upper limit of the explosion, although there will be a large number of combustible substances, but the lack of combustion-supporting oxygen, in the absence of air supplement, even in the case of open fire, will not explode for a while. Flammable solvents have a certain explosion range, and the wider the explosion range, the greater the risk.
17. Viscosity (Viscosity)
Viscosity is the internal friction resistance generated by the fluid (liquid or gas) in the flow, and its size is determined by the type of substance, temperature, concentration and other factors. Generally, it is short for dynamic viscosity, and its unit is Pa· second (Pa·s) or millipa · second (mPa·s).